Trusted Resources: News & Events
Latest announcements and gatherings
Sickle Cell Disease Approvals Include First CRISPR Gene Editing Therapy
An Ethical and Financial Obligation for Sickle Cell Disease Gene Therapy in the United States
SCDAA Teams with MedicAlert Foundation to Improve Emergency Outcomes During Sickle Cell Crises
$100,000 Grant Allows National Expansion in Expert Care of Sickle Cell Patients
SCDAA Partners With Phi Beta Sigma Fraternity
Sickle Cell Disease Association of America, Inc. and Hemanext® Inc. Form New Strategic Partnership
Sickle Cell Disease: After Years of Neglect Some Promise for Sufferers
Novel Sickle Cell Drug Causes Radical Results in End of Life Patients
A new Sickle Cell Disease Drug Holds Much Promise but Most Sufferers Won’t be Able to Afford it
FDA Approves Novel Treatment to Target Abnormality in Sickle Cell Disease
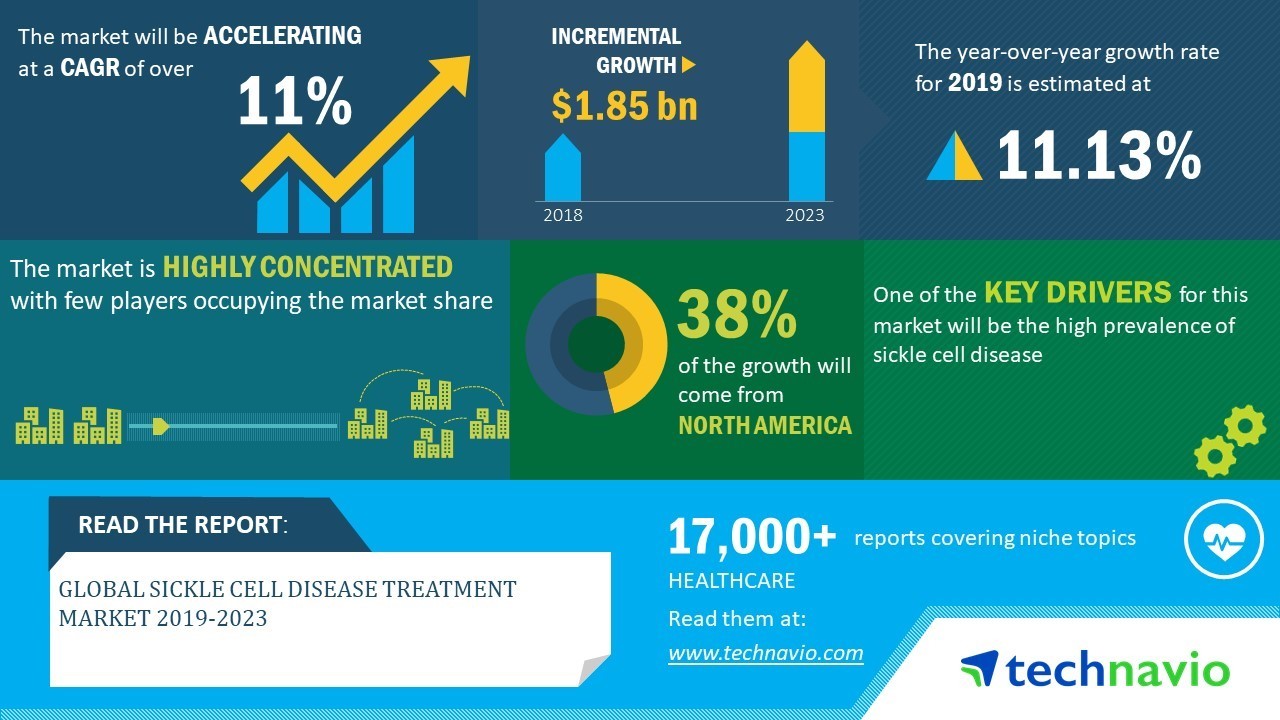
Global Sickle Cell Disease Treatment Market 2019–2023| Evolving Opportunities with ADDMEDICA and Bristol-Myers Squibb | Technavio
FDA Approves First Targeted Therapy to Treat Patients with Painful Complication of Sickle Cell Disease
Pfizer Executive Outlines Vision for Treating Sickle Cell Disease, Other Rare Diseases
GBT launches ACCEL grants program to improve access to care for people with Sickle cell disease
Emmaus Life Sciences Launches Its Commercial Co-Payment Assistance Program for Endari™
Ghana Launches Partnership With Novartis to Improve Diagnosis and Treatment of People With Sickle Cell Disease
FDA agrees accelerated approval pathway for GBT’s voxelotor
Sickle Cell Foundation Nigeria, Rhieos Develop 1st Multi-SCD Registry in Nigeria
Emmaus, a leader in sickle cell disease treatment, signs agreement with Cardinal Health to solidify distribution network for Endari™ (L-glutamine oral powder)
Siklos (hydroxyurea) now available through ProCare Pharmacy Care’s Siklos At Home program
Doris Duke Charitable Foundation awards grant to Critical Path Institute
Emmaus, a leader in sickle cell disease treatment, signs agreement with McKesson to expand distribution network for Endari™ (L-glutamine oral powder)
GBT expands sickle cell disease pipeline with worldwide licensing agreement for inclacumab for the treatment of vaso-occlusive crisis
The Links, Incorporated presents million-dollar grant to St. Jude’s Hospital
Novo Nordisk obtains licence for sickle cell disease program
California Institute For Regenerative Medicine Awards City Of Hope $5.74 Million For Severe Sickle Cell Disease Clinical Trial
University of Illinois at Chicago awarded $4.6 million NIH grant for chronic pain research
California’s Stem Cell Agency Invests in Stem Cell-Based Therapies Targeting Sickle Cell Disease and Cancer
Sickle Cell Disease Association of America, Inc. Awards Community Based Organizations With $2,033,080 for Newborn Screening Follow-Up Program
Ex-rutgers stars Devin and Jason McCourty top $1M in fundraising for charity
Emmaus Medical, Inc. Selects AmerisourceBergen to Support the Launch of EndariTM (L-glutamine oral powder)
Bluebird bio acquires Durham, NC, manufacturing site for lentiviral vector production
Cydan Raises $34M to Advance New Therapies for Sickle Cell, Other Rare Diseases
FDA Awards UNC Researcher $2M Grant to Study Kidney Disease in Sickle Cell Anemia
Matthew Porteus awarded grant for sickle cell anemia work
DDCF Awards $6 Million for Sickle Cell Disease Research
PCORI Board Approves $18 Million for Research on Sickle Cell Disease
Bioverativ and Bicycle Therapeutics Enter into Strategic Research Collaboration to Develop Therapies for Hemophilia and Sickle Cell Disease
CSL Behring to Acquire Biotech Company Calimmune and its Proprietary Stem Cell Gene Therapy Platform
Gamida Cell Announces $3.5 Million Grant from the Israeli Government
Sickle Cell Disease Association of America, Inc. Awarded $11.6 Million Continuation Grant from the Health Resources & Services Administration
GlycoMimetics Announces Pricing of Public Offering of Common Stock
Apheresis Equipment Market is expected to reach USD 3.7 billion by 2024
Global HOPE Initiative Plans $100M Pediatric Hematology-Oncology Treatment Network in Africa
U.S. Government Awards Missouri Researcher $4.3 Million to Study Sickle Cell in Teens, Adults
Exemplar Genetics Awarded Subcontract to Advance New Therapeutic Models for Sickle Cell Disease
Calimmune Expands Lentiviral Gene Therapy Pipeline Through License of Sickle Cell Disease Therapeutic Candidate
Novartis acquires US pharma research firm Selexys Pharmaceuticals
The CRISPR Era Is Here
Vaso-Occlusive Crisis: The Future of Treating Patients With Sickle Cell Disease in Emergency Departments
Corinna Schultz, MD, Shares How Pediatricians Can Discuss Sickle Cell Trait With Patients, Families
From Early Hypothesis to Clinical Data About Reducing Vaso-Occlusive Episodes with Arginine Therapy
Corinna Schultz, MD: Does Sickle Cell Trait Get Discussed?
Claudia Morris, MD, Uncovers Potential New Mechanism of Action for Hydroxyurea
Arginine Therapy Shows Positive Impacts on Sickle Cell Disease in ASH 2022 Data
MARAC Statement on Influenza, National Shortage of Oseltamivir (Tamiflu) and the Influenza Vaccine
Serious Gum Disease With Sickle Cell May Lead to Repeat Pain Crises
Lab-Grown Blood: What Is It, and How Could It Be Useful?
MARAC Encourages Clinical Research Studies
Evaluation of Ovarian Reserve, Aging and Fertility Preservation in Women With Sickle Cell Disease
SCDAA News Advisory: Partial Hold on Gene Therapy Trial
Gene therapy may help cure sickle cell disease, study says
Experimental Gene Therapy Reverses Sickle Cell Disease for Years
CTX001 Continues to Show Promise in Severe SCD
MARAC Advisory Statement: Gene Therapy & Bone Marrow Therapies
Statement on NHLBI Decision to Pause the Pilot and Feasibility Study of Hematopoietic Stem Cell Gene Transfer for Sickle Cell Disease
FDA Clears Graphite Bio to Begin Trial for Gene Therapy in Sickle Cell Disease
SCD Patients Benefit From Early Rivipansel Treatment for VOCs, New Analyses Show
Gene-Editing Treatment Shows Promise for Sickle Cell Disease
Orphan Drug Designation Granted for CSL Behring’s Investigational Plasma-Derived Hemopexin Therapy for Sickle Cell Disease
Gut Microbiome Translates Stress Into Sickle Cell Crises
Bluebird Bio Presents Data From LentiGlobin Gene Therapy Trial
Partnership to Ensure Supply of LentiGlobin, Potential Gene Therapy
Expert Opinion Hydroxyurea Could Prevent Strokes for People With Sickle Cell Disease in Lieu of Transfusions During COVID-19 Blood Supply Shortage
At 16, She’s a Pioneer in the Fight to Cure Sickle Cell Disease
A Teenager’s Breakthrough Gene Therapy for Sickle Cell Disease
ASH and FDA Unveil New Recommendations to Guide Clinical Development of Sickle Cell Disease Therapies
Gene-Edited ‘Supercells’ Make Progress In Fight Against Sickle Cell Disease
FDA Awards $2M to Phase 2 Trial of Vitamin D for Reducing Risk of SCD Respiratory Complications
Phase 3 Trial of Rivipansel in Treating SCD Pain Crisis Fails to Meet Goals, Pfizer Announces
Sickle cell trait may not increase the risk of death
Sickle Cell Patient Receives CRISPR Gene Therapy
In A 1st, Doctors In U.S. Use CRISPR Tool To Treat Patient With Genetic Disorder
Non-Invasive Prenatal Screening Test for Sickle Cell Appears Possible, Study Reports
UH Researcher Reports the Way Sickle Cells Form May Be Key to Stopping Them
Investigational Oral Inhibitor IMR-687 Shows Promising Results in Ongoing Phase 2 Clinical Trial
Cognitive Function may be Affected in Adults With Sickle Cell Disease, Study Suggests
More Online Queries in Winter Suggest Seasonal Variations in SCD Activity
More Curative Bone Marrow Transplants are Successful When Patients Given Double the Radiation Beforehand, Study Says
Johns Hopkins researchers offer new protocol to potentially cure sickle cell disease
Big Jump in Success for Sickle Cell Transplants
Could Gene Therapy Cure Sickle Cell Anemia?
Differences in Brain Oxygen Supply May Explain Silent Strokes in SCD Patients
Patients With Sickle Cell Disease may Have Lower Risk for C. Difficile
Spectra Optia Device Save Way to Treat Sickle Cell While Using Less Blood Cell Packs, Study Finds
Kids With Sickle Cell Anemia are More Sedentary Than Healthy Peers, Study Suggests
Virtual Reality Helps Reduce Pain Among Patients With Sickle Cell Disease
These patients had sickle-cell disease. Experimental therapies might have cured them
Vaso-Occlusive Pain Linked to Menstruation in Some Patients, Study Finds
Safety and early hints of benefit seen in phase 1b trial of PF-04447943
Randomized Trials Needed to Assess Benefits of Salmonella Vaccines in SCD Patients, Study Says
Toronto Neuroscientist Getting Closer to Tailored Treatments for Chronic Pain
Crizanlizumab designated FDA breakthrough therapy for potential in vaso-occlusive crisis prevention
CTX001 Earns FDA’s Fast Track Status for Treating Sickle Cell Disease
FDA grants CRISPR gene therapy fast track designation for sickle cell disease
Hospitals See No Link Between US Opioid Crisis and Patients’ Use of Treatment, Study Reports
ASH launches sickle cell disease clinical trials network to accelerate therapy development
Boston Children’s Hospital receives grant for sickle cell disease research
LentiGlobin Shows Positive Effects in Severe Sickle Cell Disease Patients, Phase 1/2 Data Reports
Gene therapy targets sickle-cell disease
Voxelotor can Promote Long-Term Benefits in Teens, Adults With SCD, Latest Clinical Data Show
Study Confirms Safe Use of Opioids for Pain Control in Sickle Cell Disease
Inexpensive sickle cell diagnostic tool shows perfect accuracy in Uganda
Early clinical trial data show gene therapy reversing sickle cell anemia
Gene therapies could transform the treatment of sickle cell disease
School staff lacks knowledge about sickle cell disease, student survey finds
Phase 1 trial to test under-the-skin injection of sevuparin in sickle cell patients
Research Team Recognized for Organ-on-a-Chip Design
Marijuana use common in sickle cell patients, highlighting need for more research, study shows
Crizanlizumab lowers pain crises in at-risk sickle cell patients, ad-hoc trial data show
Sickle cell patients in UK survey, especially those 16 to 20, voice problems with care and pain relief given
American Society of Hematology to launch sickle cell disease clinical trials network
How sickled red blood cells stick to blood vessels
Grant Recipient Uses Mobile Device Observation in Sickle Cell Pain Study
Sickle Cell Treatments can Destroy Germ Cells in Boys, Affecting Fertility in Adulthood, Study Suggests
Mount Sinai receives NIH grant to study use of inhaled corticosteroids for sickle cell treatment
L-glutamine Oral Powder Significantly Reduces Acute Complications of Sickle Cell Disease
Endari reduces pain crises, hospitalizations in sickle cell patients, phase 3 trial shows
Researchers raise funds for phase 1 trial to test medical cannabis in sickle cell disease
Music therapy helps relieve pain in adults with sickle cell disease, pilot trial suggests
GBT announces positive top-line data from part A of the phase 3 HOPE study of Voxelotor in sickle cell disease
Potential treatment targeting nitric oxide levels, olinciguat, named orphan drug by FDA
Global Blood Therapeutics (GBT) announces upcoming data presentations supporting Voxelotor SCD program
Ironwood Pharmaceuticals Announces FDA Orphan Drug Designation for Olinciguat for the Treatment of Sickle Cell Disease
Gene-Therapy Company Crispr Drops as FDA Puts Trial on Hold
Kids with sickle cell disease aren’t receiving key vaccines, Michigan study finds
Stigmatizing language in medical records affects patient care, study shows
Sickle Cell Groups, Pfizer Work to Bring African-Americans into Clinical Trials
Sickle cell trait may not contribute to stroke risk
Is Caregiver Education About Sickle Cell Trait Effective?
Healthy red blood cells owe their shape to muscle-like structures
Solution to 50-year-old mystery could lead to gene therapy for common blood disorders
FDA Approves Hydroxyurea Tablets for Pediatric Patients With Sickle Cell Anemia
ADDMEDICA receives FDA-approval for orphan drug Siklos®, first and sole hydroxyurea-based treatment for paediatric patients with sickle cell anaemia
How One Child’s Sickle Cell Mutation Helped Protect the World From Malaria
Clinical trial offers hope to adults with sickle cell disease
The impact of rare disease on family caregivers
Gamida Cell to Present Data from NiCord® Programs at the 2018 BMT Tandem Meetings
Kids Who Need Sickle Cell Meds Don’t Always Get Them
Progress in pursuit of sickle cell cure
First Sickle Cell Patient Dosed in Phase 2a of IMR-687
Hypersensitivity to Allergens May Increase Risk of Acute Chest Syndrome in Sickle Cell Anemia Children
University researchers develop more complete model of sickle cell
Hydroxyurea Treatment in Men with SCA Leads to Drop in Total Sperm Count, Study Shows
Sickle Cell Treatment Endari Now Available in the United States
GBT Receives FDA Breakthrough Therapy Designation for Voxelotor for Treatment of Sickle Cell Disease (SCD)
La Jolla Pharmaceutical company announces initiation of pivotal clinical study of LJPC-401 in patients with beta thalassemia
Emmaus Life Sciences, Inc. to present results of phase 3 study of endariTM (L-glutamine oral powder) at 59th American Society of Hematology annual meeting
Review Board Finds Global Blood’s Sickle Cell Disease Therapy Voxelotor Is Safe
Sickle cell patient with severe anemia rapidly improves with voxelotor, case study shows
Alzheimer’s Treatment Memantine Shows Promise in Treating Sickle Cell Disease
Investigational Therapy Altemia Achieves Main Endpoints in Pediatric Sickle Cell Trial
Georgia Universities Join NIH-funded National Study of Bone Marrow Transplant for SCD
Hydroxyurea linked to ‘significant, rapid’ reduction of sperm count
SCA Therapy Hydroxyurea Doesn’t Boost Malaria Risk in Sub-Saharan Africa, Study Finds
Fighting sickle cell disease by looking back to babyhood
National Sickle Cell Disease Poll of African Americans Dispels Long-Held Views
Imara Reports Favorable Preclinical and Phase 1 Data on IMR-687 in Sickle Cell Disease
Computer models provide new understanding of sickle cell disease
‘Natural’ Gene Mutation May Offer Way of Treating Sickle Cell Disease, Study Says
Quest Diagnostics Launches Genetic Test to Assess Risk for Sickle Cell Anemia, Other Inherited Disorders
FDA Approves Glutamine Powder for Sickle Cell Disease
Researchers Identify Genetic Predictors of Sickle Cell Anemia-Related Complications
Global Blood Therapeutics Receives EMA PRIME Designation for GBT440 for the Treatment of Sickle Cell Disease (SCD)
Chip-based Models Mimic Organs to Advance Understanding of Sickle Cell Disease
Small chips, big impact: MSU researcher studies cardiovascular, sickle cell disease
ASH to develop clinical guidelines to improve care for people with sickle cell disease
Only 21% of Kids with ADHD and SCD Are Treated for Attention Deficit
New Pre-transplant Treatment Regimen Improves Survival of Kids with Sickle Cell Disease, Trial Shows
Researchers Develop Test for SCA to be Used in Low-Resource Settings
Multidisciplinary Care Team Greatly Reduces Risk in Pregnant SCD Patients in Low-income Countries
FDA Advisory Committee Recommends Approval of Endari™ from Emmaus Life Sciences for the Treatment of Sickle Cell Disease
New Method of Creating Healthy Stem Cells Could Potentially Improve Treatment of Sickle Cell Anemia
Imara’s IMR-687 for SCD Receives FDA Rare Pediatric Disease Designation
Phase 3 Trial Recruiting to Test Rivipansel for Vaso-Occlusive Crisis in SCD
Sickle Cell Disease and Its Toll Compared in Different Age Groups in Study
Prolong Pharma’s Sanguinate Shows Promise in Reverting Shape of Red Blood Cells in SCD
New Research Suggests SANGUINATE™ Reduces the Number of Sickled Red Blood Cells in Patients with Vaso-Occlusive Crisis
MaxCyte, Inc. to Present Positive Preclinical Data for Sickle Cell Disease
First systemic evidence for safety of tPA in stroke patients with sickle cell disease
Young Sickle Cell Patients Who Don’t Take Medication Have Lower Quality of Life
Older Blood Used in Transfusions May Be Harmful to Adult Sickle Cell Patients, Review Finds
Sickle cell gene linked to elevated risk of developing kidney failure
In France, Boy Becomes First Sickle Cell Disease Patient to be Treated With Gene Therapy
Sickle Cell Anemia Toddlers Benefit from Maximal Hydroxyurea Doses, Study Finds
bluebird bio Announces Publication of Case Study on First Patient with Severe Sickle Cell Disease Treated with Gene Therapy in The New England Journal of Medicine
Study Suggests Ways of Improving Newborn Screening for Sickle Cell Disease
Sickle cell trait may confound blood sugar readings among African-Americans
A multiple drug approach to preventing sickle cell crisis
World’s Largest Sickle Cell Disease Stem Cell Library Underway
Diabetes Drug, Metformin, Suggested as ‘Breakthrough’ Treatment for Sickle Cell Anemia
SPCI Launched the SCOT (Sickle Cell Omega-3 Treatment) Trial in the United States with an Investigator Meeting Held December 16-17 in Atlanta
SUSTAIN Clinical Trial Results Show Crizanlizumab Reduced Sickle Cell–Related Pain Crises
Sickle Cell Disease Research Shows Progress in Preventing Related Complications and Death
Step toward gene therapy for sickle cell disease
Stem cell gene-editing method may be breakthrough for sickle cell research
Environmental, genetic factors may predict longevity in sickle cell disease
CRISPR deployed to combat sickle-cell anaemia
Methadone provides pain relief for kids with sickle cell
Rare patients with sickle cell disease live nearly twice national average
Effort set to help sickle cell patients manage meds
BCL11A-based gene therapy for sickle cell disease passes key preclinical test
Researchers ID key drivers of heart complications in sickle cell anemia
Discovery could help treatments for sickle cell disease
Study challenges view that sickle cell trait increases mortality risk
Pregnant Women with Sickle Cell Disease, Especially SS-type, at Risk of Complications
Long-term opioids may not be best pain management option for all sickle cell patients
Hydroxyurea improves lung function in children with sickle cell disease
Genetic Treatments for Sickle Cell
Pediatric sickle cell study stopped early due to positive results
New Sickle Cell Disease Research Shows Improved Patient Outcomes
Sickle cell disease cure in sights of UAB Stem Cell Institute
100 Years But Only One Drug: Sickle Cell Patients Wait For Help
Black Americans With Sickle Cell Trait At Increased Risk Of Kidney Disease
SCDAA 2023 National Abstract Competition
10th Annual Warriors Convention – Sickle Cell Community Consortium
Chief Patient Officer Summit
Sickle Cell Disease Camp – JWCF at RoundUp River Ranch
2023 NHLBI Annual Sickle Cell Disease Research Meeting
NHLBI Annual Sickle Cell Disease Research Meeting
SCDAA Masterclass Speaker Series: All Things Considered – SCD Treatment: A Personal Choice
Class of 2021-2023 Graduation Book
Cayenne Wellness Center’s 15th Annual Sickle Cell Disease Educational Summit
10th Annual Walk with the Stars – Sickle Cell Disease Association of America, Inc.
Congressional Briefing: Comprehensive Sickle Cell Disease Care in a Time of Innovation
Chiltern 50 Challenge
6th Annual Chicago Sickle Cell Summit
Society of Pediatric Psychology Fall Virtual Conference
Show Love, Give Blood for National Sickle Cell Awareness Month 2023
Annual National Sickle Cell Disease Association of America (SCDAA) Convention
P.O.W.E.R ECHO Project Community Health Worker (CHW) Training
Sickle Cell Community Consortium: Mental Health & Wellness Initiative
Clinical Trials 101: Everything You Need to Know to Get Started – Somebody To Talk To Zoom Session
SCDF: BabySteps Parent Education Meeting
SCANJ: 11th Annual Statewide Sickle Cell Disease Symposium
Sickle Cell Community-Wide Support & Empowerment Group (Nov)
Minority Health Counts: Building a More Equitable Community Summit
65th American Society of Hematology (ASH) Annual Meeting & Exposition
Sickle Cell Foundation of Palm Beach County & Treasure Coast Sickle Cell-ebration Awareness Walk 2023
SCANJ Holiday Party North with Kenta Klaus
Sickle Cell Community Consortium: Sickle Cell Holiday Expo 2023
SCANJ Holiday Party South with Kenta Klaus
Sickle Cell Community-Wide Support & Empowerment Group (Dec)
SCDAA Spring Community Health Worker Training
Virtual Sickle Cell Disease Policy Forum
P.O.W.E.R ECHO Project Community Health Worker (CHW) Training – 3/21/24
8th Annual Leadership Summit and General Assembly of Patients, Caregivers & CBOs
P.O.W.E.R ECHO Project Community Health Worker (CHW) Training – 4/18/24
P.O.W.E.R ECHO Project Community Health Worker (CHW) Training – 5/19/24
5th Annual World Sickle Cell Day 24-Hour Sickle Cell-a-Thon
SCDAA And MedicAlert Pilot Program Application
4th Annual Virtual Caregivers Summit – Sickle Cell Community Consortium
Sickle Cell Cure Brings Mix of Anxiety and Hope
Sickle Cell Management During the Blood Crisis With Edward Ivy, MD, MPH
Edward Ivy, MD, MPH: A Comprehensive Perspective on Sickle Cell Disease
Regina Hartfield Is Now the CEO and President of SCDAA
Q&A With Regina Hartfield, New CEO of the SCDAA
Sickle Cell Disease Association of America, Inc. Names New CEO & President
These Sisters With Sickle Cell Had Devastating, and Preventable, Strokes
Nobel Prize Spotlights Sickle Cell’s Disproportionate Impact on African Americans
Ron Cook: James Franklin Bracing for ‘Heartache’ of College Football Season
Sister of Illinois’ first coronavirus victim also dies from virus. Quarantine prevents family from mourning together.
Patricia Frieson was more than the 1st COVID-19 death in Illinois. She was their sister.
28-Year-old Sickle Cell Survivor Becomes Medical Doctor
‘I Have Sickle Cell, but Sickle Cell Doesn’t Have Me’: While Raising Awareness, Chicago Man, 25, Dies From Illness
What It’s Like to Live With Sickle-Cell
Sickle cell disease is complex on its own, but black men with the illness battle its stigmas and stereotypes too
Too many children live too far from sickle cell treatment they need
WNY Girl Gets First Pediatric Bone Marrow Transplant for Sickle Cell in Buffalo
Students With Sickle Cell Disease Stay on Track With Help From St. Louis Children’s Hospital
Dr. Doris Wethers, 91, on Front Lines against Sickle Cell, Dies
Sickle Cell Treatment ‘Life-Changing’ for Brockton Brothers
Surprise of a lifetime: Devin McCourty gives away two Super Bowl LIII tickets
McCourty twins praise aunt who lost battle with sickle cell disease for ‘defying the odds’
The ‘Voice of Lagos’ is silent: Entertainer Tosyn Bucknor dies at 37
Devin and Jason McCourty Honored by NFLPA for Community Work
Jordin Sparks Wants You to Know Your Sickle Cell Status
Williams excels on soccer field while playing with sickle cell trait
Matteson girl, 8, with sickle cell hosts party, blood drive to help others
Charlotte 13-Year-Old With Sickle Cell Plays Lead Role in Broadway’s ‘Lion King’
Family of late MMA fighter Rondel Clark starts foundation to raise awareness about extreme weight cutting
Sickle cell advocate wins fight for high-dose opioids
Henrico twins with sickle cell disease take on Washington
Chief executive of the Sickle Cell Society is awarded an OBE
The Life, Death, and Dream of a Research Diversity Crusader
Queens’s boy, 13, among advocates, doctors in Albany to champion new sickle cell bill
A Britain’s Got Talent contestant revealed how she turned to singing following the death of her son
Sickle Cell Disease Association of America, Inc. Hires New President & CEO
Hospital Playroom Transformed Into Romantic Restaurant For Parents Of Kids With Bone Marrow Transplants
Jordin Sparks Remembers Sister Who Died of Sickle Cell Complications: ‘She Was So Strong’
9-year-old violin prodigy defeats strokes, paralysis; raising money for bone-marrow transplant
Shakir Cannon, advocate for minority health, dies
Camp Jumoke helping kids cope with sickle cell disease
Teen with sickle cell disease takes class trip to Europe with Loyola Medicine’s help
Sickle Cell Disease Association Announces National Child Ambassador
Patriots’ Devin McCourty raises awareness about sickle cell disease
My medical school lesson was tinged with racism. Did that affect how I treated a sickle cell patient years later?
Siri saves sick girl from Harvey floodwaters
Prodigy’s death shines light on slow progress against sickle cell disease
Pfizer’s Kevin Williams to Pen Health Column on Sickle Cell Disease for the Black Press
Sickle cell cure is real, as this Kansas patient proves
Mixing Music and Medicine: Meet Grammy-Nominated Producer Nana Kwabena
Artist Panteha Abareshi Opens Up About Sickle Cell Disease and Expressing Her Pain Through Art in “The Girl Who Loves Roses”
Health or education? The daily dilemmas of a student with sickle cell anaemia
Ty Montgomery says he’s not worried about sickle cell trait
Cardinals’ Bruce Arians: WR John Brown carries sickle-cell trait
PROFILE: Asiata, 90-year-old sickler who has 5 children and has gone to Mecca 13 times
Flying doctor takes to the skies after sister’s death
SCDAA Statement About Gene Therapy Approval
SCDAA News Advisory: SCDAA Statement on Exa-cel Gene Therapy
MARAC Statement: Update About COVID
MARAC Statement: Health Insurance Coverage for Hematopoietic Stem Cell Transplant for Sickle Cell Disease from HLA-matched Sibling Donor (MSD HCT)
MARAC Statement: Crizanlizumab (Adakveo)
MARAC Advisory Statement: Update on Penicillin Shortages
23andMe, Morehouse School of Medicine and the Sickle Cell Foundation of Georgia Inc. launches the Sickle Cell Carrier Status Awareness Program
SCD C.A.R.E.S. Consortium
Why genetic engineering experts are putting a spotlight on Victoria Gray’s case
New York Senate Bill 1839 and Assembly Bill 6430—Sickle Cell Disease Detection and Education Program
MARAC Statement: Penicillin Shortage
Standing Up for Sickle Cell
CMO Speaks: Fertility Care and SCD
California Campaign Hopes to Raise Awareness About Sickle Cell Trait
The Orphan Drug Act Turns 40: NORD Celebrates Its Impact on Rare Diseases
The Warrior Network – Sickle Cell Community Consortium
Sickle Cell Disease and Sickle Cell Trait – SCDAA Brochure
MARAC Advisory Statement: Immunizations
MARAC Advisory Statement: Monkeypox
SCDAA Releases Comments on the CDC’s Opioid Guidelines
The Sickle Cell Disease Association of America (SCDAA) Recognized for Leadership
MARAC Advisory: COVID-19 and Sickle Cell Disease
Support the CDC’s World Sickle Cell Day Social Media Campaign!
International Association of Sickle Cell Nurses and Professional Associates (IASCNAPA) Scholarships
SCDAA Celebrates Black History Month: Miles Davis
Red Cross Declares First-Ever Blood Crisis Amid Omicron Surge
MARAC Advisory Statement: Update About COVID-19
COVID-19 Vaccines for Children and Teens
Former U.S. President Jimmy Carter and First Lady Rosalynn Commend Sickle Cell Foundation of Georgia, Inc.
SCDAA News Advisory: Salmonella and Sickle Cell Disease
MARAC Advisory Statement: Update About COVID-19 Vaccines
A Proclamation on National Sickle Cell Awareness Month, 2021
MARAC Advisory Statement: COVID-19 Update
MARAC Advisory Statement: Monoclonal Antibodies Against SARS-CoV-2
ASH President: No Medical Merit to Sickle Cell Trait to Explain In-Custody Deaths
ASH Position on Sickle Cell Trait
How a Genetic Trait in Black People Can Give the Police Cover
Angels in Heaven Memorial Booklet
COVID-19 Vaccine Communication Toolkit for Community-Based Organizations
Public Review of CureSCi Common Data Elements
MARAC Advisory Statement: COVID-19 Vaccines
1st Patients to Get CRISPR Gene-Editing Treatment Continue to Thrive
Why I Will Be Getting the COVID-19 Vaccine
Readout From the First Lady’s Roundtable on Improving the Lives of Americans Living With Sickle Cell Disease
Proclamation on National Sickle Cell Disease Awareness Month, 2020 – By the President of the United States of America
SCDAA MARAC Position on 2020 School Reopening
What People With Sickle Cell Disease Need to Know About COVID-19
Coronavirus, Racial Disparity in Sickle Cell Disease
Greensboro 7-Year-old With Sickle Cell Disease Raises Money for Kids Like him
Sickle Cell Disease: All you Need to Know
Peer-to-Peer Mentoring Program – SCDAA
Smartphone App May Help Assess Anemia Using Eyelid Pictures
COVID-19 Health Alert for Patients and Caregivers – SCDAA
Vitamin D Supplements May Reduce Pain-related Emergency Room Visits in Children With SCD
What’s Inside My Medicine Cabinet?
How My Parents Set Me Up for Success in Sickle Cell Management
MARAC Advisory Statement Regarding SCD Patients during the time of “Reopening” the U.S. Economy
In Support of Rare Disease Patients Impacted by COVID-19, NORD Launches Premium and Limited Medical Relief Program
Information About COVID-19 for Sickle Cell Disease Patients
Supermarkets Offer Special Hours for Older Shoppers
NORD Launches Financial Assistance Program for Rare Disease Community Members Impacted by COVID-19
Healthwell Foundation Sickle Cell Disease Fund
Sickle Cell Disease Association of America Extends its COVID-19 Emergency Fundraiser
Sickle Cell Disease and COVID-19: An Outline to Decrease Burden and Minimize Morbidity (Adapted for Sub-Saharan Africa)
COVID-19 Health Alert for People With Sickle Cell Disease and Their Caregivers (Adapted for Sub-Saharan Africa)
NORD/EURORDIS-Rare Diseases Europe Joint Statement on COVID-19 and Orphan Drug Legislation
Coronavirus/COVID-19 & Sickle Cell Disorder (Sickle Cell Foundation Nigeria)
Rumor, Disparity and Distrust: Why Black Americans Face an Uphill Battle Against COVID-19
GBT Supports Sickle Cell Patients During COVID-19 Pandemic
COVID-19 Resources for Non-Profit Leaders and the Community | National Organization for Rare Disorders
Severe Blood Shortage Due To Coronavirus Outbreak
A Health Note From Dr. Biree Andemariam, SCDAA Chief Medical Officer
Howard University College of Medicine Receives Grant to Support Youth With Sickle Cell Disease
SCD Patients Receiving Hydroxyurea in the U.S. Still Face Many Challenges
HHS Secretary Alex Azar Touts White House Efforts to Fight Sickle Cell Disease
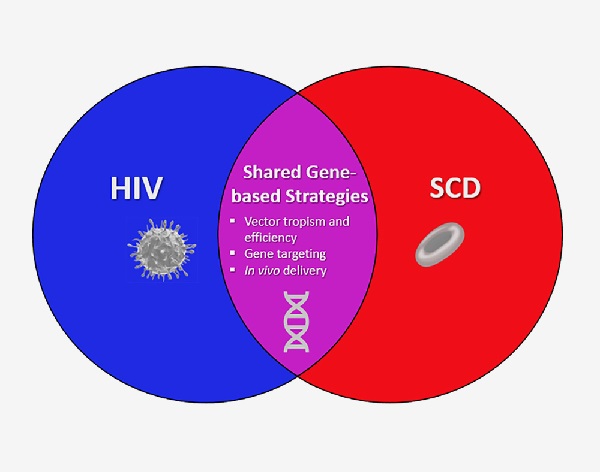
NIH Launches new Collaboration to Develop Gene-Based Cures for Sickle Cell Disease and HIV on Global Scale
CDC Expands SCD Data Collection Program to 7 More US States in Effort to Improve Services
Presidential Message on National Sickle Cell Disease Awareness Month 2019
5 Myths and Facts About Sickle Cell Disease (SCD)
NHLBI Stepping Up Efforts to Apprise SCD Patients of Therapies and Trials
‘Sickle Cell Speaks’ Campaign Raises Awareness with Aim of Eroding Stigmas, GBT and Partners Say
CIRM and NHLBI Collaborating to Fund Cell and Gene Therapies for Sickle Cell Disease
NFL Broadcaster Solomon Wilcots and Emmaus Life Sciences Kick Off “Sideline Sickle Cell” Campaign during National Minority Health Month
Identifying Outcomes for Sickle Cell Disease Clinical Trials is Aim of coreSCD
Congo Delegation Visits Howard University to Bolster Collaborations on Sickle Cell Disease
Representative Johnson Files Four Sickle Cell Disease Related Bills
ATS Releases Guidelines for Home Oxygen Therapy in Children With Sickle Cell Disease
Dr. Wayne A. I. Frederick launches ‘Run to Cure Sickle Cell’ campaign
Sickle Cell: Call The Midwife Shines Spotlight on Disease
Healthcare advocates aim to better inform community on sickle cell realities
ASH offers early look at updated SCD guidelines: Experts formulated >50 recommendations on sickle cell disease
The Sickle Cell Treatment Act (Bill S. 2465) Passed the Senate!
Greenville program tries team approach to improve lifespan of sickle cell disease patients
Pfizer rare disease introduces Council for Change to further help SCD patients
Novartis teams up with recording artist and actress Jordin Sparks and SCDAA to launch Generation S, an inspiring new sickle cell storytelling project
Initiative helping sickle cell patients
Siklos®, the first and only hydroxyurea-based treatment for pediatric patients with sickle cell anemia, now available in retail pharmacies
Patients frequently turning to cannabis to treat symptoms of sickle cell disease
Statement from FDA Commissioner on agency’s efforts to advance development of gene therapies
Joining hands: SCDAA and Emmaus medical partner to fight sickle cell disease
Spearheading change on World Sickle Cell Day
New drugs promise hope this World Sickle Cell Day
Everyday Heroes: A view beyond the blood
Red Cross seeks more blood with #MissingType campaign
Sickle cell patients suffer as disparities in care and research persist
Bioverativ and Sangamo announce FDA acceptance of IND application for gene-edited cell therapy BIVV003 to treat sickle cell disease
Re-Authorization of the Sickle Cell Treatment Act passes Through the House of Representatives
CRISPR could end sickle cell disease, but signing up black patients for clinical trials will be a hard sell
New Jersey may start a confidential sickle cell trait registry
Boston public schools agree to recognize sickle cell disease as disability
How a Man and Woman, Both with a Sickle Cell Anemia Mutation, Had a Healthy Child
Sickle cell patients, families and doctors face a ‘fight for everything’
The Cellie Coping Kit helps sick kids manage the stress of treatment
Why the NHS needs more black people to give blood
University Hospitals program uses music to teach about sickle cell (photos, video)
‘Every time it’s a battle’: In excruciating pain, sickle cell patients are shunted aside
Opioid Abuse Backlash Could Hurt Sickle Cell Patients’ Medication Needs, Some Fear
Sickle-Cell Patients See Hope in CRISPR
The Upside of Bad Genes
Why selective marriage can’t help people with Sickle Cell disorder —Expert
Pfizer and National Newspaper Publishers to Raise Awareness About Sickle Cell Disease
Pfizer and the national newspaper publishers association collaborate to raise awareness of sickle cell disease and need for improved patient care
The National Sickle Cell Advocate Network (NSCAN)
When Cracking Down On Opioids Means Tougher Access For Sickle Cell Patients
Our healthcare system abandons adult sickle cell patients
Sickle Cell Disease and Cold Weather: Dos and Don’ts
Sickle Cell in Primetime: How A Character Reveal on Shonda Rhimes’ ‘Grey’s Anatomy’ Renewed My Hope
To improve your experience on this site, we use cookies. This includes cookies essential for the basic functioning of our website, cookies for analytics purposes, and cookies enabling us to personalize site content. By clicking on 'Accept' or any content on this site, you agree that cookies can be placed. You may adjust your browser's cookie settings to suit your preferences. More Information
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this.





 +myBinder
+myBinder