DISCLAIMER
The information and materials accessed through or made available for use on any of our Sites, including, any information about diseases, conditions, treatments, or medicines, are for informational purposes only. The Content is not intended to be and is not a substitute for professional medical advice, diagnosis, or treatment, and your participation on our Sites does not create a healthcare professional-patient relationship. You should consult a doctor or other qualified health care professional regarding any questions you have about your health or before making any decisions related to your health or wellness. Call your doctor or 911 immediately if you think you may have a medical emergency.compose your message
message sent
email sent successfully
Trusted Resources: News & Events
Latest announcements and gatherings
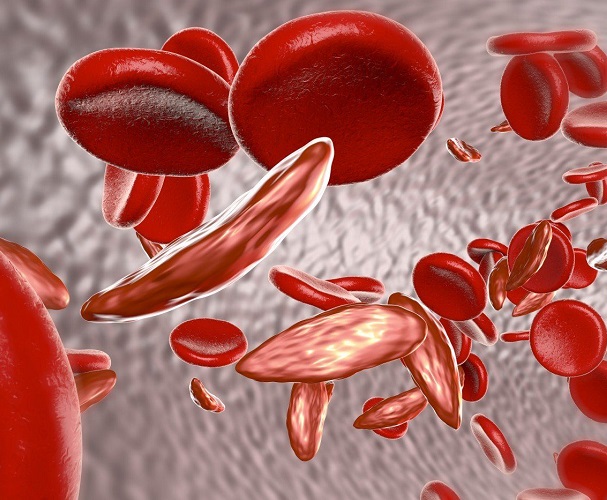
Crizanlizumab designated FDA breakthrough therapy for potential in vaso-occlusive crisis prevention
Crizanlizumab (SEG101), Novartis‘ investigational compound for sickle cell disease (SCD), received breakthrough therapy designation from the U.S. Food and Drug Administration for its potential to prevent vaso-occlusive crises (VOCs).
The FDA designation was granted based on promising data from the company’s multicenter, randomized, double-blind, 52-week, Phase 2 SUSTAIN clinical trial (NCT01895361).
“Painful sickle cell crises matter because they can disrupt patients’ lives, and often require hospital visits and medical attention,” Samit Hirawat, MD, head of global drug development at Novartis Oncology, said in a press release. “We look forward to working closely with the FDA over the coming months toward making crizanlizumab, a therapy that has the potential to prevent sickle cell pain crises, available in the U.S. as soon as possible.”
 +myBinder
+myBinderRelated Content
-
news & eventsA new Sickle Cell Disease Drug Holds Much Promise but Most Sufferers Won’t be Able to Afford itThe regulatory approval of a groundbreak...
-
education & researchOrphan Drugs for Sickle Vaso-occlusion: Dawn of a New Era of Targeted TreatmentWhile an orphan disease in the USA, sick...
-
education & researchHospitalization for Acute Pain in Sickle Cell Disease: Changes in Clinical Parameters and Factors Predicting Hospita...Introduction: Acute vaso-occlusive crisi...
-
news & eventsGut Microbiome Translates Stress Into Sickle Cell CrisesA new study shows how chronic psychologi...
-
news & eventsHow sickled red blood cells stick to blood vesselsOne of the most common complications o...
-
videos & visualsVaso occlusive Crisis Pain Assessment & Managementhttps://www.youtube.com/watch?time_conti...
-
news & eventsResearch Team Recognized for Organ-on-a-Chip DesignSCD is a group of genetic disorders that...
send a message
To improve your experience on this site, we use cookies. This includes cookies essential for the basic functioning of our website, cookies for analytics purposes, and cookies enabling us to personalize site content. By clicking on 'Accept' or any content on this site, you agree that cookies can be placed. You may adjust your browser's cookie settings to suit your preferences. More Information
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this.
Support for this site is provided by

This platform is made possible through a partnership with the Sickle Cell Disease Association of America, Inc. (SCDAA) and its member organizations. SCDAA's mission is to advocate for people affected by sickle cell conditions and empower community-based organizations to maximize quality of life and raise public consciousness while advancing the search for a universal cure.




