DISCLAIMER
The information and materials accessed through or made available for use on any of our Sites, including, any information about diseases, conditions, treatments, or medicines, are for informational purposes only. The Content is not intended to be and is not a substitute for professional medical advice, diagnosis, or treatment, and your participation on our Sites does not create a healthcare professional-patient relationship. You should consult a doctor or other qualified health care professional regarding any questions you have about your health or before making any decisions related to your health or wellness. Call your doctor or 911 immediately if you think you may have a medical emergency.compose your message
message sent
email sent successfully
Trusted Resources: News & Events
Latest announcements and gatherings
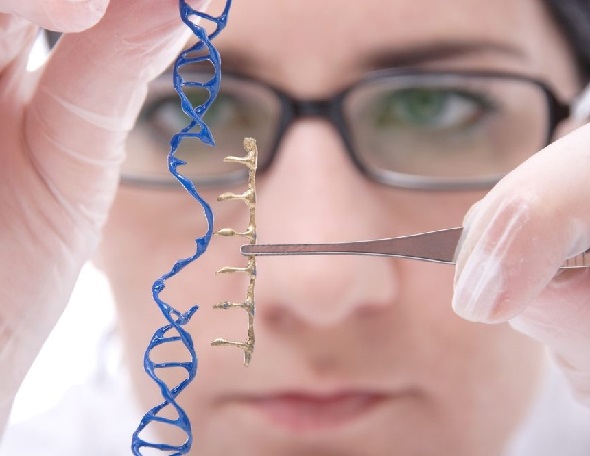
LentiGlobin Shows Positive Effects in Severe Sickle Cell Disease Patients, Phase 1/2 Data Reports
Bluebird bio presented new positive results from the ongoing Phase 1/2 HGB-206 clinical trial evaluating the effectiveness and safety of investigational gene therapy LentiGlobin in patients with severe sickle cell anemia.
The company had previously revealed findings from the trial at the end of last year and earlier this year. The new results reflect data collected through September 14.
Results were introduced at the recent 60th Annual Meeting of the American Society of Hematology in San Diego, under the abstract name “Current Results of LentiGlobin Gene Therapy in Patients with Severe Sickle Cell Disease Treated Under Refined Protocol.”
 +myBinder
+myBinderRelated Content
-
education & researchBarriers and facilitators to research participation among adults, and parents of children with sickle cell disease: ...Patient recruitment into sickle cell dis...
-
news & eventsExperimental Gene Therapy Reverses Sickle Cell Disease for YearsA study of an investigational gene thera...
-
news & eventsSickle Cell Patient Receives CRISPR Gene TherapyMany human diseases can be traced back t...
-
news & eventsPartnership to Ensure Supply of LentiGlobin, Potential Gene TherapyBluebird Bio extended a partnership with...
-
news & eventsNHLBI Stepping Up Efforts to Apprise SCD Patients of Therapies and TrialsWide interest in a CBS 60 Minutes story ...
-
news & eventsIn France, Boy Becomes First Sickle Cell Disease Patient to be Treated With Gene TherapyA 13-year-old boy with sickle cell disea...
-
education & researchCulturally competent strategies for recruitment and retention of African American populations into clinical trialsPurpose: To identify successful recrui...
send a message
To improve your experience on this site, we use cookies. This includes cookies essential for the basic functioning of our website, cookies for analytics purposes, and cookies enabling us to personalize site content. By clicking on 'Accept' or any content on this site, you agree that cookies can be placed. You may adjust your browser's cookie settings to suit your preferences. More Information
The cookie settings on this website are set to "allow cookies" to give you the best browsing experience possible. If you continue to use this website without changing your cookie settings or you click "Accept" below then you are consenting to this.
Support for this site is provided by

This platform is made possible through a partnership with the Sickle Cell Disease Association of America, Inc. (SCDAA) and its member organizations. SCDAA's mission is to advocate for people affected by sickle cell conditions and empower community-based organizations to maximize quality of life and raise public consciousness while advancing the search for a universal cure.




